NUCLEOLIN AND NUCLEOPHOSMIN AS EXPECTED TARGETS FOR CATIONIC PEPTIDES, INDUCING TUMOR CELL APOPTOSIS
DOI: https://doi.org/10.18454/jbg.2018.3.8.1
Lushnikova A.A.1 , Ponkratova D.A.2 , Kostarev A.V.3, Rudakova A.A. 4 , Andreev S.M.5 , Baryshnikova M.A.6
1, 2, 4, 6 N. N. Blokhin National Medical Research Centre of oncology, Moscow, Russia
3 Lomonosov Moscow State University, Moscow, Russia
5 Immunology Institute of FMBA of Russia, Moscow, Russia
* Correspodning author (LAN21@yandex.ru)
Received: 23.07.2018; Accepted: 06.08.2018; Published: 17.08.2018
NUCLEOLIN AND NUCLEOPHOSMIN AS EXPECTED TARGETS FOR CATIONIC PEPTIDES, INDUCING TUMOR CELL APOPTOSIS
Research article
Abstract
Multifunctional proteins nucleolin (NCL, C23) and nucleophosmin (NPM, B23) are the most abundant nucleolar phosphoproteins with chaperone activities. Nucleolin is over-expressed also on tumor cell surface. The proteins regulate the most of important cell functions, they are considered now as the cell proliferation markers and potential targets for cancer treatment Objective is a screening for some cationic peptides as an expected ligands for NCL/NPM and novel agents with selective cytotoxicity in human cutaneous melanoma cell lines. Three original cell lines mel IS, mel H and mel Ki obtained from the lymph node metastasis of the patients with metastatic cutaneous melanoma were used as a models. To evaluate binding between NCL, NPM and peptides under study molecular docking was used. The data obtained allow to characterize tested cationic peptides as a ligands for NCL and NPM triggered subsequent tumor cell apoptosis.
Keywords: cutaneous melanoma, cell lines, nucleolin, nucleophosmin, cationic peptides, apoptosis induction.
Лушникова A.A. 1 , Понкратова Д.А.2 , Костарев A.В.3 , Рудакова A.A. 4 , Андреев С.М.5 , Барышникова M.A. 6
1, 2, 4, 6 НМИЦ онкологии им.Н.Н.Блохина, Москва, Россия
3 Московский государственный университет имени М. В. Ломоносова, Москва, Россия
5 Государственный научный центр “Институт иммунологии”, Москва, Россия
* Корреспондирующий автор (LAN21@yandex.ru)
Получена: 23.07.2018; Доработана: 06.08.2018; Опубликована: 17.08.2018
НУКЛЕОЛИН И НУКЛЕОФОЗМИН КАК ПРЕДПОЛАГАЕМЫЕ МИШЕНИ ДЛЯ КАТИОННЫХ ПЕПТИДОВ, ИНДУЦИРУЮЩИХ АПОПТОЗ ОПУХОЛЕВЫХ КЛЕТОК
Научная статья
Аннотация
Многофункциональные белки нуклеолин (NCL, C23) и нуклеофозмин (NPM, B23) – мажорные ядрышковые фосфопротеины с шаперонной активностью. Нуклеолин гиперэкспрессируется также и на поверхности опухолевых клеток. Эти белки регулируют важнейшие клеточные функции, являются маркерами клеточной пролиферации и рассматриваются в качестве потенциальных мишеней для молекулярно-направленной терапии рака. Цель исследования – скрининг некоторых катионных пептидов – предполагаемых лигандов NCL/NPM, обладающих избирательной токсичностью в отношении клеток метастатической меланомы кожи человека. Три оригинальных линии mel IS, mel H и mel Ki были получены из метастазов в лимфатических лимфоузлах пациентов с меланомой кожи. Для оценки молекулярных взаимодействий между NCL, NPM и катионными пептидами использовали парный докинг. Полученные результаты позволяют охарактеризовать тестированные пептиды как лиганды нуклеолина и нуклеофозмина, индуцирующие апоптоз опухолевых клеток.
Ключевые слова: меланома кожи, клеточные линии, нуклеолин, нуклеофозмин, катионные пептиды, индукция апоптоза.
1. Introduction
Nucleolin (NCL, C23) and nucleophosmin ((NPM, B23) are the most abundant nuclear phosphoproteins with chaperone activities. Currently, they are considered as markers of the cell proliferation and as potential targets for cancer treatment [1]. These chaperone proteins regulate DNA transcription, RNA translation, chromatin remodeling, cell signaling, angiogenesis, and carcinogenesis. Many of the NCL/NPM functions are realized through the interactions with other protein molecules involved in carcinogenesis, ribosome biogenesis, DNA repair, regulation of genome stability, cell division and survival, epithelial–mesenchymal transition, invasion, differentiation, and apoptosis. Nucleolin is overexpressed on the surface of cancer cells, but the NCL level is usually minimal on the surface of normal cells. In addition, NCL and NPM are also expressed in the cell nucleus and cytoplasm. The differential expression of these proteins in tumor and normal cells allows using them as a targets for selective inhibition of tumor growth, in particular, in hard-to-treat cancers. Metastatic cutaneous melanoma is one of the most common types of skin cancer, the progression of which does not allow specific long-term treatment. Therefore, the study of diagnostic biomarkers, as well as predictive and prognostic markers of melanoma is a global challenge [2]. An important fundamental and application problem is the analysis of mechanisms of metastasis and inhibition of this process [2]. Objective is to study cationic peptides as novel agents with selective cytotoxicity for melanoma, as well as possible ligands for nucleolin and nucleophosmin.
2. System and methods
Three original cell lines - mel IS, mel H and mel Ki – were obtained from the lymph nodes of the patients with disseminated cutaneous melanoma. Using PCR, BRAF-V600E mutations were found in mel IS and mel H cell lines, while in mel Ki cell line, wild-type BRAF kinase was found. Normal human fibroblast H1036 and Wi38 lines were used as a control. All the cells were cultured in the standard RPMI 1640 medium supplemented with 10% fetal bovine serum. Immunohistochemical detection and western blot analysis with monoclonal antibodies (Abcam) were used to assess the expression levels of NCL/NPM. Using MTT assay (test of survival), seven cationic peptides (CP), enriched with arginine and lysine (R/K), with molecular weights from 1090 to 2338 Da and charges from 4+ to 16+ were analyzed, (Table 1). These peptides were synthesized by solid-phase method by the strategy based on using of Fmoc protective groups. Then they were diluted in sterile distilled water at a concentration of 4-0.25 μg/ml and added to the culture flasks for 2-3 days. The cytotoxicity of peptides was also assessed in mini-chambers with melanoma cell culture (Eppendorf, Germany) in presence Cy5- labeled peptide NC811, followed by visualization of the cells under fluorescence microscopy. To assess the binding between NCL and NPM and the peptides under investigation, molecular docking was used [3]. The data obtained allow characterizing the tested cationic peptides as NCL and NPM ligands inducing apoptosis of tumor cells.
2.1. Algorithm
First, using the MTT assay and visualization of apoptosis under fluorescence microscopy, we identified a group of cationic peptides with a high selective cytotoxicity to human cutaneous melanoma cells. The next step was to characterize the expression of NCL and NPM in melanoma cells, co-localization of these proteins and tested peptides, and to analyze the mechanisms of cell death induced by these peptides. Finally, using bioinformation resources, including molecular docking, the cytotoxic cationic peptides were investigated as true ligands for overexpressed NCL and NPM proteins.
2.2. Application
An integrated approach using original cell models and a collections of cationic peptides followed by bioinformatic analysis of molecular interactions between NCL / NPM chaperone proteins and pre-tested peptides can be the basis for selective antitumor therapy with cationic peptides targeting nucleolin.
3. Results
The use of cationic peptides with a specific molecular structure to induce selective cell death would solve the problem of melanoma resistance to standard therapy. The tested cationic peptides have a number of advantages: (1) much lower toxicity to normal cells compared with chemotherapeutic agents; (2) a rapid cell membrane permeability; (3) interaction with surface and intracellular targets of tumor cells, mainly with overexpressed nucleolin molecules on the surface of cell membranes. Seven cationic peptides enriched with Arg (R) and Lys (K) were selected for their antiproliferative activity after 2-3 days of incubation with the above-mentioned melanoma cell cultures. Main physical and chemical properties of peptides are given in Table 1.
Table 1 – Cationic peptides having antiproliferative activity against melanoma cells in vitro
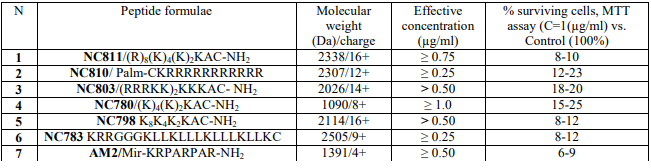
Melanoma cell lines with different mutant status of BRAF tyrosine kinase were used as models for studying the expression of NCL/NPM and cytotoxicity of peptides (Figure 1).
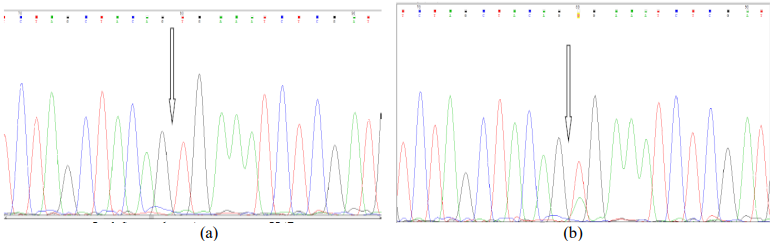
Figure 1 – Fragments of sequences coding wild type and mutant V600E BRAF:
(a) sequence of exon 15 of the BRAF gene in cell lines of fibroblasts H1036 and melanoma mel Ki (wt) obtained by Sanger sequencing, position c.1799T is indicated;
(b) Sequencing of exon 15 of the BRAF gene in cells of line mel IS (mutation), nucleotide substitution c.1799T>A (p.V600E) is indicated
Figure 2 – Levels of NCL (a) and NPM (b) differential expression in cutaneous melanoma mel IS lines, immunohistochemical staining of cells, x200; (c) western-blotting using monoclonal antibodies against NCL/NPM and actin
It was found that all the studied cell lines were sensitive to the tested peptides at concentrations of 0.5-4 μg/ml, (Table 1). The survival rate observed in the control lines of fibroblast Wi-38 and H1036 was about 100%. To study the mechanisms of selective cell death of melanoma cells in vitro, we used Cy-5 fluorescently labeled peptide NC811, its molecular structure is shown in Fig. 3.
Figure 3 – Molecular structure of NC811-Cy5, A ─ alanine, K ─ lysine, R ─ arginine, C ─ cysteine, Cy5 ─ cyanine 5
Cationic peptides enriched with Arg (R) and Lys (K) have low toxicity, penetrate the membranes of melanoma cells, and reach the nucleolus due to the interaction with molecules of membrane nucleolin and then with nucleophosmin. After 2-4 hours of the cell incubation with aqueous solution of Cy5-NC811 (C = 1 μg/ml), it was localized in the nucleus together with labeled proteins NCL/NPM and p53. Apoptosis, inhibiting the proliferation of melanoma cells, was detected by Höchst and DAPI (4', 6-Diamino-2'-phenylindole dihydrochloride) cell staining, (Figure 4).
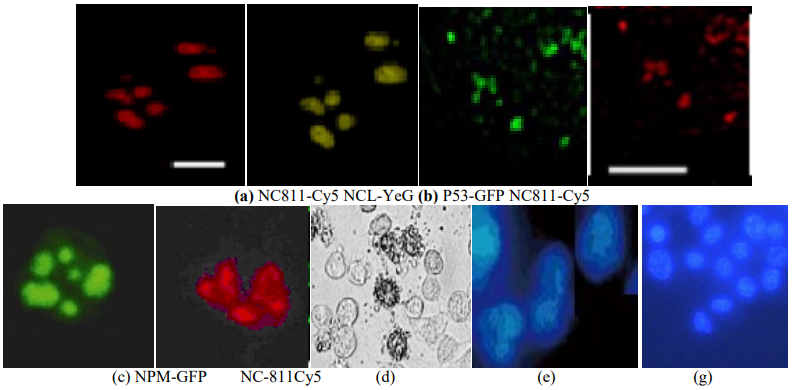
Figure 4 ─ Apoptosis in melanoma cells confirmed by Höchst 33342 or DAPI staining after 2 hours of incubation of cells with NC811-Cy5; (a) co-localization of fluorescently labeled cationic peptide NC811-Cy5 and NCL in the nuclei of mel IS cells, scale = 7 µm; (b); co- localization of labeled p53 and NC811-Cy5 in the nuclei of mel H cells, scale = 25 µm (c); co- localization of NC811-Cy5 and labeled NPM in the nuclei of mel IS cells; x400; apoptosis of tumor cells, phase contrast (d); staining of mel IS cells with Höchst-33342, x200 (e); and DAPI, x1000 (g)
However, apoptosis was not observed in normal fibroblasts in H1036 or Wi-38 cell lines. Flow cytometry with fluorescently labeled caspases was used to assess the mechanisms of selective melanoma cell death. Tumor cells of mel IS line were incubated with cationic peptide AM-2 during 24 hours followed by flow cytometry. The results show a significant activation of caspases 3, 8, 9 labeled with FITC, which is induced by the peptide: 5.6 times for caspase 3, 13.3 times for caspase 8, and 4 times for caspase 9 (Figure 5).
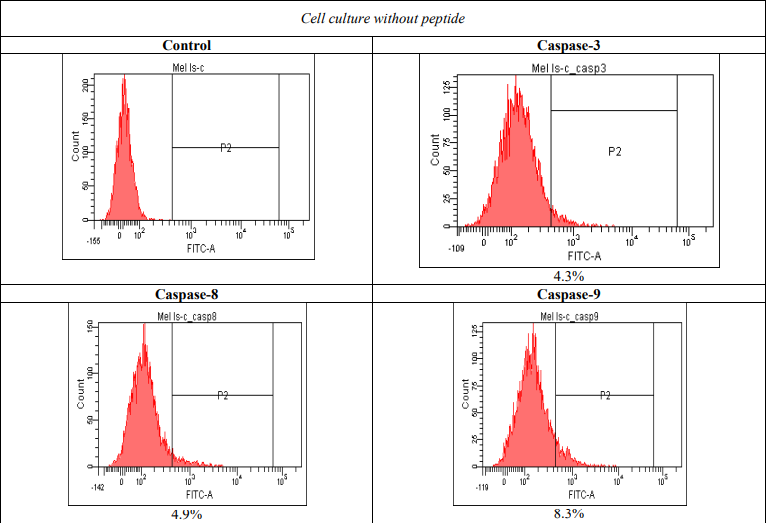
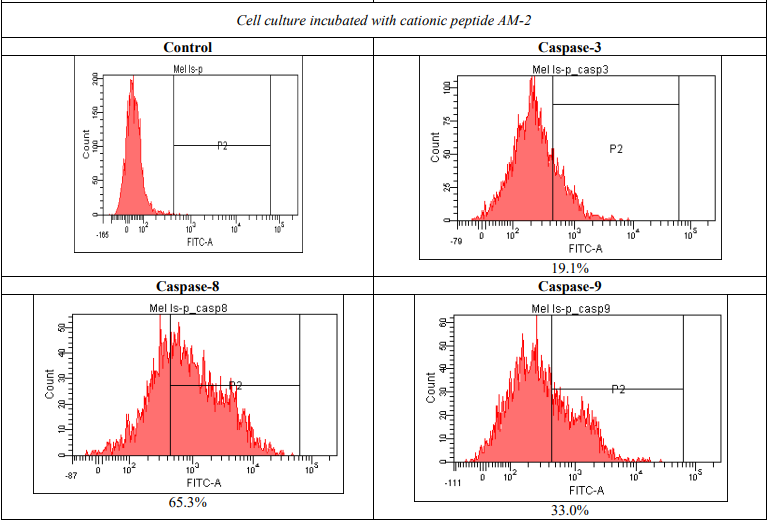
Figure 5 – Activation of caspases due to the incubation with cationic peptide AM-2 (1 µg/ml)
The activation of caspases leads to reorganization of the cytoskeleton and cell disintegration stimulating apoptosis. We assume that cationic peptides (ligands) bind to their target (receptor) – membrane NCL and activate initiatory caspases 8, 9, 10, then caspases 3, 6 with subsequent inactivation of cellular functions. The destruction of cell structures during apoptosis and fragmentation of chromosomal DNA result in inactivation of the CAD enzyme complex (carbamoyl-phosphate synthetase 2, aspartate transcarbamoylase, and dihydroorotase) combined with inhibitor of CAD – ICAD, in blockage of CAD release by caspase 3 , DNA fragmentation and degradation of nucleosomes. The inactivation of enzymes involved in DNA repair includes poly(ADP-ribose)- polymerases, or PARP, which were first described as caspase substrates. The PARP family is involved in the repair of DNA damage and chromatin remodeling due to poly-ADP-ribosylation of histones. During apoptosis, caspase-3 inactivates PARP-1 and inactivate DNA repair. The destruction of DNA is also enhanced by the inactivation of topoisomerase II by caspases. The nucleolus functions as a compartment that is sensitive to cellular stress and isolates p53, which is released into nucleoplasm upon DNA damage. The mechanisms of protein sequestration into the nucleolus includes interaction of proteins with resident nucleolar proteins NCL and NPM nucleolar localization signal (NoLS) enriched with R/K amino acid residues.
4. Molecular docking for assessment of interactions between cationic peptides, nucleolin/NLC dimers and nucleophosmin/NPM pentamers
Binding between some cationic peptides and their suspected targets – NCL and NPM – was analyzed by molecular docking using Maestro 11 software [4], [5]. In spite of the prevailing localization of nucleolin in the nucleoli, it is known that the phosphorylated and/or glycosylated forms of NCL are also expressed on the cell surface. Cell surface, or receptor, nucleolin is an important regulator of carcinogenesis processes, mainly by binding to ligands. Previously, we identified a number of cationic peptides enriched with Arg/Lys that serve as potential ligands of the receptor NCL and form clusters in the active center of the NCL dimer, followed by its inactivation and degradation of tumor cells by the apoptosis mechanism. [2], [3]. Molecular docking confirms a possibility of binding between NCL and peptide molecules , since stable hydrogen bonds with NH2-groups of amino acid residues in the peptide molecule were revealed (Fig 6).
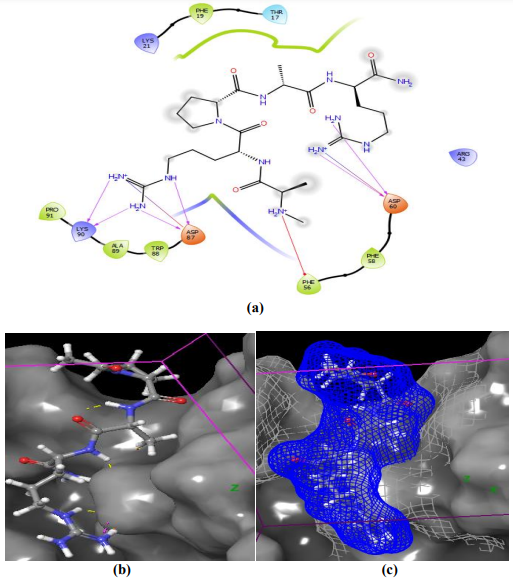
Figure 6 – AM-2 peptide binding to NCL involves Asp 87, Lys 90 , Phe 56, Asp 60, 87, Lys 90 in the NCL dimer (a); cluster AM-2 is positioned in the binding domain of the active center of the NCL dimer receptor (b); AM-2 ligand interaction with NCL surface in the coordinate grid (c)
The results of docking showed that key amino acids important for the interaction of NLC with the ligand are Asp, Glu (q-, pH7.0), Arg , Lys, (q+, pH7.0), Thr (q 0, pH7.0), localized in the active center of NCL. They bind to different peptide domains forming stable hydrogen bonds with NH2-groups of amino acid residues in the peptide molecule (Figure 6, for example). Nucleophosmin acts mainly in the form of pentamer and hydrogen bonds are more often formed between amino acids Glu (q-, pH7.0), Arg (q+, pH7.0), Gly (q0, pH7.0) localized in the active center and some NH2-groups in the peptide molecule (Figure 7, a-c).
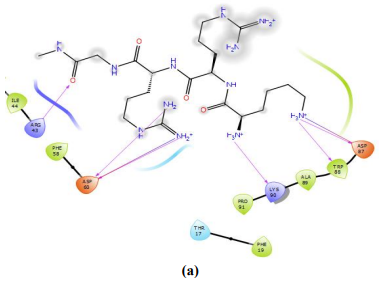
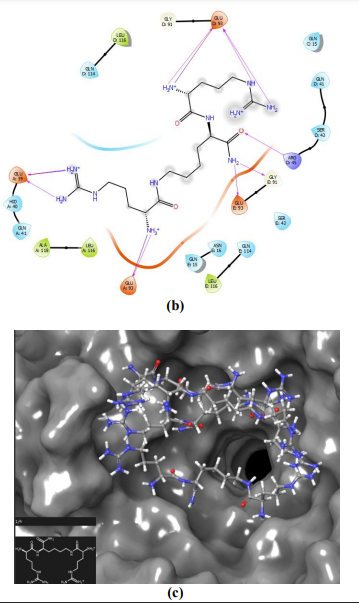
Figure 7 – Cationic peptide NC783 binding with NPM active center. Glu93 forms the maximum number of hydrogen bonds with the best score between NPM and ligand (a, b). Interaction between the ligand –NC783 - and NPM, spatial arrangement of the ligand on the surface of NPM pentamer with the maximum score is shown (c)
5. Discussion
Molecules of NCL and NPM contain a nucleolar localization signal domain (NoLS) enriched with lysine and arginine containing repeats, or motifs, that interact with noncoding RNAs transcribed from intergenic rDNA sequences. It is known that the N-terminal domain of the nucleolin molecule similar to high-mobility groups (HMG), interacts with 4 motifs containing residues of glutamate and aspartate, which alternate with the residues of lysine. These amino acid sequences with pH7.0) within DNA molecule. Nucleolin molecule contains 4 central RNA-binding domains (RBD1-4) and C-terminal domain enriched with arginine/R and glycine/G – GAR (Glycine Arginine Rich). RNA-binding domains of nucleolin specifically interact with the 5' external transcribed spacer (ETS) of processed rRNA and specifically bind to RNA, and GAR-domain specifically binds to DNA and non-specifically – to RNA [6]. The interaction between NCL and cationic peptides is the most likely starting point for inducing selective antiproliferative action of cationic peptides. The binding of peptide molecules to nucleolin that is overexpressed on the surface of tumor cells and further release of suppressor protein p53 associated with NCL induces apoptosis causing the previously found melanoma cell death [7, 8]. Apoptosis was detected in all the three model melanoma lines using Höchst or DAPI cell staining. However, apoptosis did not affect normal skin fibroblast in H 1036 and Wi-38 lines. This is due to a low level of expression of membrane NCL in normal cells, so the inactivation of the receptor and the release of p53 followed by apoptosis do not occur. According to the results of the MTT assay, cationic peptides with dendritic structure have the highest inhibitory activity. The molecular docking confirms this pattern, but a detailed analysis of the apoptosis induction process in melanoma cells has not yet been completed. The paper analyzes the inhibition of melanoma cell proliferation by tested cationic peptides. These results, as well as earlier ones, regarding the induction of apoptosis in other tumor lines confirm the role of peptides as ligands of cell surfase nucleolin and nucleophosmin. The tested cationic peptides represent promising antitumor agents that require further study.
Supplementary materials
Does not apply.
Funding
The work was supported by State Contract N 114112440122, Ministry of Health.
Acknowledgement
Does not apply.
Conflicts of interest
None declared.
References
Jia W. New perspective of physiological and pathological functions of nucleolin / Yao Z., Zhao J., Guan Q. and others // Life Science. – 2017. – V. 186. – P.1-10.
Fujiki H. Cell-surfase nucleolin acts as a central mediator for carcinogenic, anti-carcinogenic, and disease-related ligands / Watanabe, T., Suganuma M. //. J. Cancer Res Clin Oncol. – 2014. – V. 140. – P. 689-699.
Koutsioumpa M. Cell surface nucleolin as a target for anti-cancer therapies / Papadimitriou E. // Recent pat Anticancer Drug Discov. – V. 9 – № 2. – P. 137-52.
SiteMap User Manual Copyright © 2009 Schrödinger, LLC. Schrödinger Press
Glide User Manual Copyright © 2015 Schrödinger, LLC. Schrödinger Press
Mei Q. Regulation of DNA replication-coupled histone gene expression / Huang J, Chen W. // Oncotarget. – 2017. – V. 8. – P. 95005–95022.
Ponkratova D.A. Study for anti-tumor activity of cationic peptides on the molel cell lines / Lushnikova A.A., Morozova L.F., Andreev S.M. and others // Russian Journal of Biotherapy. – 2017. – V. 16. – P. 64-65.
Lushnikova A.A. The mechanisms of antitumor toxocoty in a number of cationic peptides / Ponkratova D.A., Rudakova A.A., Andreev S.M. and others // Modern Science. – 2017. – № 11. – P. 124-126.
