ФУНКЦИЯ ГИППОКАМПА И РАННЕЕ ВЫЯВЛЕНИЕ БОЛЕЗНИ АЛЬЦГЕЙМЕРА С ИСПОЛЬЗОВАНИЕМ МАШИННОГО ОБУЧЕНИЯ
DOI: https:/doi.org/10.18454/jbg.2022.1.17.1
Ziani A.1 *, Yakoubi O.2, Ziani A.3
1, 2, 3 University of Science and Technology Houary Boumedienne, Algiers, Algeria
* Correspodning author (zianianakim[at]gmail.com)
Received: 04.04.2022; Accepted: 19.04.2022; Published: 19.05.2022
HIPPOCAMPAL FUNCTION AND EARLY DETECTION OF ALZHEIMER’S DISEASE USING MACHINE LEARNING
Research article
Abstract
As of today, there is no cure for Alzheimer’s disease, which is a progressive and irreversible neurodegenerative illness of brain tissue that has no known cure. It causes a progressive and irreversible loss of mental functions, most notably memory, as well as other symptoms. The most significant challenge is that there is no definitive gold standard for diagnosing Alzheimer’s disease at the present time. This dementia should be diagnosed early, and we wish to underline the crucial function played by the hippocampus in this early detection of Alzheimer’s disease to aid in the identification of this type of dementia. We constructed a model that could accurately distinguish between non-demented persons and mildly demented patients.
Keywords: hippocampus, SVM, Classification, Alzheimer’s Disease, Machine Learning, AI.
Зиани А.1 *, Якуби О.2, Зиани А.3
1, 2, 3 Университет науки и технологий им. Хуари Бумедьен, Алжир, Алжир
* Корреспондирующий автора (zianianakim[at]gmail.com)
Получена: 04.04.2022; Доработана: 19.04.2022; Опубликована: 19.05.2022
ФУНКЦИЯ ГИППОКАМПА И РАННЕЕ ВЫЯВЛЕНИЕ БОЛЕЗНИ АЛЬЦГЕЙМЕРА С ИСПОЛЬЗОВАНИЕМ МАШИННОГО ОБУЧЕНИЯ
Научная статья
Аннотация
На сегодняшний день не существует лекарства от болезни Альцгеймера, которая представляет собой прогрессирующее и необратимое нейродегенеративное заболевание мозговой ткани, лечение которого неизвестно. Это вызывает прогрессирующую и необратимую потерю психических функций, в первую очередь памяти, а также другие симптомы. Наиболее серьезной проблемой является то, что в настоящее время не существует окончательного золотого стандарта диагностики болезни Альцгеймера. Эта деменция должна быть диагностирована на ранней стадии, и мы хотим подчеркнуть решающую функцию, которую играет гиппокамп в этом раннем выявлении болезни Альцгеймера, чтобы помочь в выявлении этого типа деменции. Мы построили модель, которая могла бы точно различать людей, не страдающих психическими расстройствами, и пациентов с легкой степенью психического расстройства.
Ключевые слова: гиппокамп, SVM, классификация, болезнь Альцгеймера, машинное обучение, искусственный интеллект.
1. Introduction
Alzheimer’s disease (AD) is the most common type of dementia, a degenerative brain ailment that primarily affects older adults. It is the most frequently seen cause of de- mentia in humans [1], [2]. AD affects around 26 million individuals globally now, including 100,000 in Algeria [3], according to epidemiological data. Doctors must be able to pinpoint precisely which imaging alterations occurred during a pathology, which is not always a straightforward process.
As a result, diagnostic tools are required. Our work aims to emphasize the critical role of the hippocampus in this early detection of Alzheimer’s disease by developing a powerful yet simple tool capable of automatically classifying a brain’s MRI between non- and mild-demented states with the highest possible accuracy and then analyzing which MRI pixels were most influential in the classification and comparing them to hippocampus area pixels. This enables health care facilities to alert patients who are in the early stages of dementia. and stimulate future study in this area.
Our initial objective will be to set a decision limit precisely at the point at which a brain MRI begins to show signs of dementia. Due to their efficiency on this type of assignment, we have changed our focus to SVM and Decision Tree classifiers to accomplish this binary classification.
2. Methods
In the field of health informatics, machine learning has been widely used in bioinformatics, medical imaging, medical informatics, and public health. The authors in [9] Pre-affirmed the importance of Hippocampal region on the diagnosis. They proposed a machine learning method to diagnosis alzheimer’s disease using Hippocampal region volume. ITK-SNAP, a semi-automatic segmentation software, was used to calculate volume information, and a data set was created based on age, gender, diagnosis, and right and left hippocampus volume values, in this work we aim to classify entire MRI images and then extract the region of interests.
In order to train an SVM classifier for Alzheimer’s disease diagnosis, Zhang et al. [10] suggested extracting specified tissue volumes. The selection of relevant brain regions and learning dimensionality transformation can be found by combining the marginal fisher analysis with norm based multi-kernel learning [11]. For the diagnosis of AD/MCI, deep learning networks have recently been investigated [12].
Classification is a supervised learning technique which learns a function from training a data set that consists of input features/attributes and categorical output [1]. This function will be used to predict a class label of any valid input vector. The primary objective of classification is to use machine learning algorithms in order to achieve the best prediction accuracy possible [4]. The Support Vector Machine (SVM) algorithm proposed by Vapnik is a modern approach for solving the pattern recognition problems. The SVM algorithm maps the sample points into a high dimensional feature space to seek for an optimal separating hyperplane through max-imizing the margin between two classes. SVM algorithm is the supervised machine learning algorithm. The SVM algorithm is successfully used for the different classification problems in various applications. The SVM classifiers on the base of the SVM algorithm have been applied for credit risk analysis, medical diagnostics, text categorization, information extraction, etc [5].
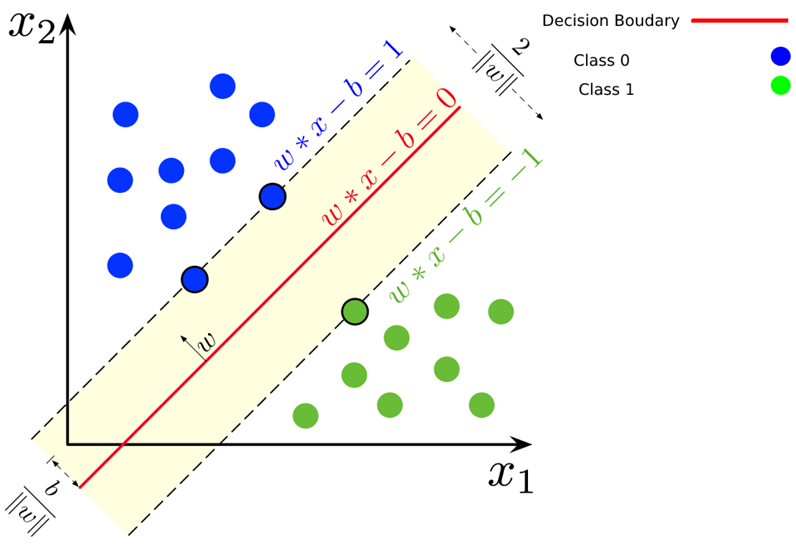
Fig. 1 – Linear decision boundary of SVM
Kernels are used in Support Vector Machines to map the learning data (non-linearly) into a higher dimensional feature space where the computational power of the linear learning machine is increased [6].
Radial basis function (RBF) is a general-purpose kernel used when there is no prior knowledge about the data.

Where in (1), σ is the variance and hyperparameter and ∥x − y∥ is the Euclidean (L 2 − norm) distance between two points x and y.
Polynomial Kernel represents the similarity of vectors in a feature space over polynomials of the original variables.

Where d is the degree of the kernel.
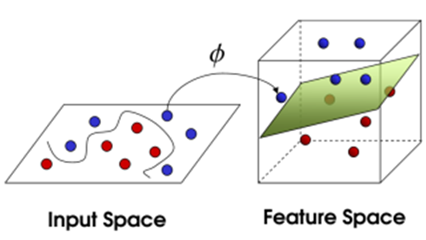
Fig. 2 – Examples of applying a kernel to an input space
Most machine learning algorithms depend on the certain hyper parameters to work and in the general case there is no clear way to calculate these perimeters so for most of the cases this task ends up being a very iterative step while training the best model. In this study we used the grid search method to obtain these parameters. The grid search is originally an exhaustive search based on a defined subset of the hyper-parameter space. The hyper-parameters are specified using minimal value (lower bound), maximal value (upper bound) and number of steps. There are three different scales that can be used: linear scale, quadratic scale and logarithmic scale. The performance of every combination is evaluated using some performance metrics. [7].
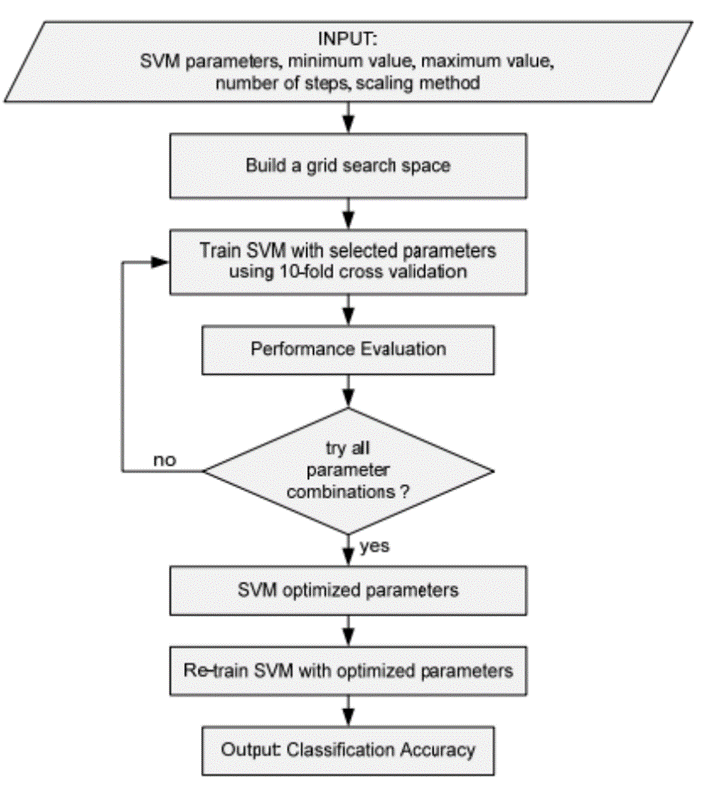
Fig. 3 – Process of finding parameters using grid search
3. Results
The data used in this study was obtained from an online Kaggle challenge of MRI brain images. The images are black and white with a standard dimension of 176x208 pixels with a total size of about 2120 images separated into 1696 training 424 test examples. The dataset consists into two classes of brain images: Non-Demented and Mild-Demented. Each image is a 2D MRI cross section of the brain as shown in Figure 4.
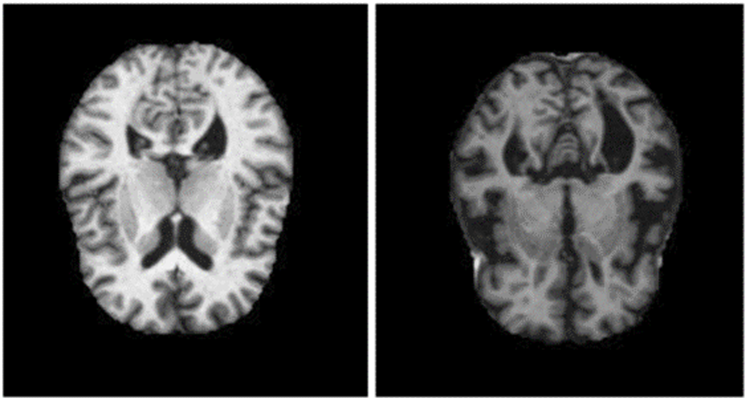
Fig. 4 – Non and mild demented MRI brain images from the dataset
Images came with the brain cross-section centered in the image and with the background cropped out. For making the model faster, we chose to re-scale the images with a 0.5 scaling factor. The input vector for is a flatten version of the pixel matrix of each image. the shape of the vector is (1,9152).
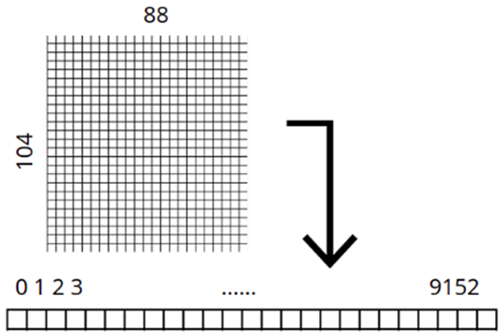
Fig. 5 – Image shape transformation applied to images
After realizing the test and training our model, the hyper-parameters chosen are shown in Table1.
Table 1 – Hyper Parameter after performing a GridSearch
|
Hyper-parameter |
Kernel |
C |
Gamma |
|
Value |
RBF |
65 |
0,001 |
We obtained a 1.0 accuracy on the SVM model. This means that every test example was classified correctly. The confusion matrix is shown in Fig 6.
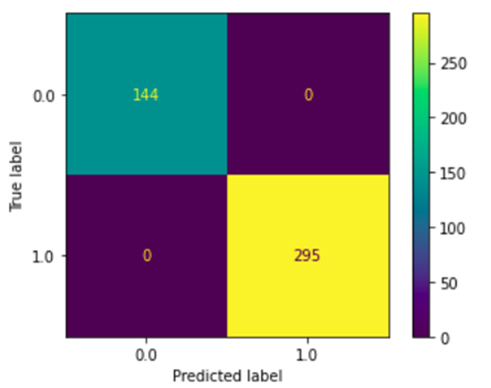
Fig. 6 – Confusion matrix of the SVM model
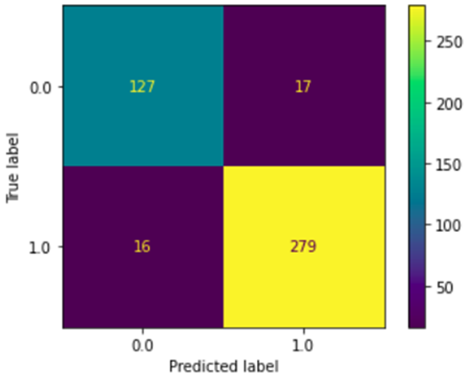
Fig. 7 – Confusion matrix of the DecisionTree model
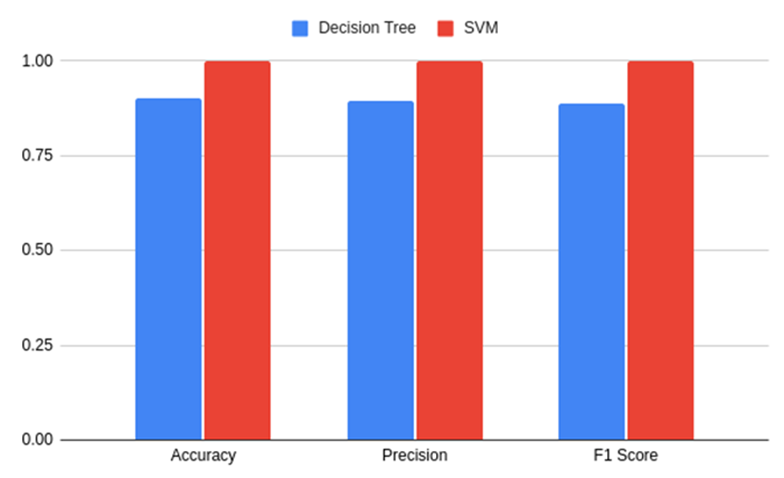
Fig. 8 – Performance metrics on trained models
Table 2 – Performance metrics on trained models
|
Performance metrics |
Accuracy |
Precision |
F1 Score |
|
SVM |
1 |
1 |
1 |
|
Decision Tree |
0,90 |
0,89 |
0,88 |
To explain this high accuracy, we trained a Decision Tree model with the same training and testing dataset. We obtained a 0.92 accuracy score. The Fig 7 shows the confusion matrix of this trained model.
Following the training of a Decision Tree model, we were able to extract and then locate the most influential pixels in the classification tasks. This work was completed by reshaping the vector image to its original 104x88 proportions using Adobe Illustrator. Figure 9 depicts the principal area in which these pixels are distributed.
A critical brain area for learning and memory, the hippocampus is particularly vulnerable to damage during the early stages of Alzheimer’s disease (AD). Recent data suggest that de- creased neurogenesis in the adult hippocampus is a critical early event in the evolution of Alzheimer’s disease (AD) [9], which is a degenerative brain disease.
4. Discussion
The portion of the brain illustrated in Fig 9 is quite similar to the hippocampus a particularly vulnerable area to damage during the early stages of Alzheimer’s disease region of the human brain. Hopefully, this serves as a timely reminder of the essential function that this structure plays in the diagnosis and/or classification of Alzheimer’s disease.
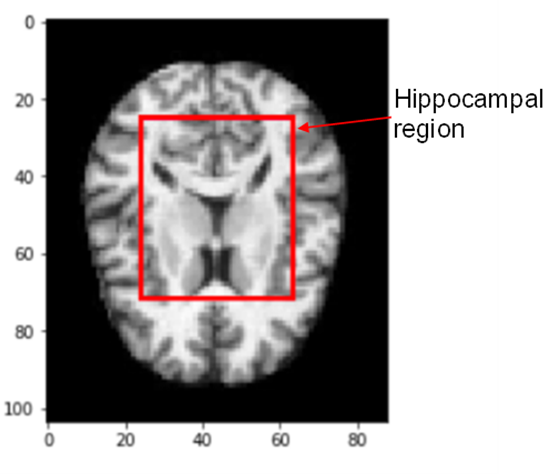
Fig. 9 – Main area where most influential pixels are located
5. Conclusion
In this study, we created an SVM classifier model that is capable of recognizing early Alzheimer’s disease dementia in a brain magnetic resonance imaging (MRI) image. The results confirmed the model’s ability to perform well on this type of data. The Decision Tree model, on the other hand, provides an explanation for the accuracy discovered. In addition, we discovered that the hippocampus plays a significant role in the classification of Alzheimer’s disease, which was previously known but neglected in past few years. This research has the potential to make important contributions to the field of computer-assisted diagnosis of Alzheimer’s disease in the near future by reminding the importance of this area in Alzheimer’s disease.
|
Conflict of Interest |
Конфликт интересов |
|
None declared. |
Не указан. |
Список литературы
Masters Colin L. Alzheimer’s Disease [Electronic resource] / L. Masters Colin et al.// Research Profiles at Washington University School of Medicine – 20 Mar. 2019 – URL: profiles.wustl.edu/en/publications/alzheimers-disease. (accessed: 03.04.2022)
Dubois B. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group / B. Dubois et al. // The Lancet Neurology – Volume 20 – Issue 6, 2021 DOI: 10.1016/S1474-4422(21)00066-1.
Alzheimer Disease [Electronic resource] // International World Alzheimer Report 2009. – URL: https://www.alz.co.uk/research/files/WorldAlzheimerReport.pdf (accessed: 03.04.2022)
Arevalo-Rodriguez I. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI) / I. Arevalo-Rodriguez, N. Smailagic, M. Roqué i Figuls et al. // Cochrane Database of Systematic Reviews – 2015, Issue 3 – Art. No.: CD010783. DOI: 10.1002/14651858.CD010783.pub2.
Demidova L. Improving the Classification Quality of the SVM Classifier for the Imbalanced Datasets on the Base of Ideas the SMOTE Algorithm / L. Demidova, I. Klyueva // ITM Web Conf. – 10 (2017) – 02002 DOI: 10.1051/itmconf/20171002002
Vapnik V.N. Statistical Learning Theory / V.N. Vapnik – John Wilcy and Sons, 1998
Liashchynskyi P. Grid Search, Random Search / P. Liashchynskyi, P. Liashchynskyi // Genetic Algorithm: A Big Comparison for NAS, 2019 DOI: 10.48550/ARXIV.1912.06059
SVM Parameter Optimization using Grid Search and Genetic Algorithm to Improve Classification Performance / TELKOMNIKA (Telecommunication Computing Electronics and Control) – 2016 – ISSN: 1693-6930
Gokce U. Hippocampal atrophy-based Alzheimer’s disease diagnosis via machine learning methods / U. Gokce, M. Ozturk // Journal of Neuroscience Methods – Volume 337, 2020 – 108669 DOI: 10.1016/j.jneumeth.2020.108669.
Zhang D. Multimodal classification of Alzheimer’s disease and mild cognitive impairment / D. Zhang, Y. Wang, L. Zhou et al. // Neuroimage – vol. 55 – 2011 – p. 856
Cao P. Nonlinearity-aware based dimensionality reduction and over-sampling for AD/MCI classification from MRI measures / P. Cao, X. Liu, J. Yang et al. // Computers in Biology Medicine – vol. 91 – 2017
Ortiz A. Ensembles of Deep Learning Architectures for The Early Diagnosis of Alzheimer’s Disease / A. Ortiz, J. Munilla, J. M. Górriz et al. // International Journal of Neural Systems – vol. 26 – 2016 – p. 1650025
